The team of Prof. YANG Wei consecutively published achievements on muscle aging related competing endogenous RNA networks, Gouqi/human milk-derived nanovesicles (GqDNVs) inhibiting muscle atrophy, and the association between muscle wasting and all-cause mortality risk
Recently, YANG’s team (W. Y) published four publications about the involvement of competing endogenous RNA (ceRNA)networks in muscle aging process, exosomes inhibiting muscle atrophy and the association of muscle wasting and mortality risk in Journal of Nanobiotechnology (IF5y = 11.5), Journal of Cachexia, Sarcopenia and Muscle (IF5y = 10.7) and Mechanisms of Ageing and Development (IF5y = 5.3).
The Yuxiao LIAO (assistant research fellow), Zhao PENG (assistant research fellow), Zitong MENG, Xiaolei ZHOU and Huan-huan ZHOU are first authors, respectively. Prof. Wei YANG is corresponding author. The Department of Nutrition and Food Hygiene, Hubei Key Laboratory of Food Nutrition and Safety, Tongji Medical College, Huazhong University of Science and Technology is the first corresponding affiliation. University of Tübingen (Germany) is co-operative affiliation. These works are supported by the National Key Research and Development Program of China (Grant No. 2022YFC3600600 from W.Y), Natural Science Foundation of Hubei Province (Grant No. 2021CFB313 from W.Y) and Nutrition and Care of Maternal & Child Research Fund Project of Biostime Institute of Nutrition & Care (Grant No. 2021BINCMCF051 from W. Y).
1:Gouqi-derived nanovesicles (GqDNVs) inhibited dexamethasone-induced muscle atrophy associating with AMPK/SIRT1/PGC1α signaling pathway (Journal of Nanobiotechnology, 2024).
With the increasing trend of global aging, sarcopenia has become a significant public health issue. Goji berry, also known as “Gou qi zi” (枸杞子) in China, is a traditional Chinese herb that can enhance the structure and function of muscles and bones. Otherwise, previous excellent publications illustrated that plant-derived exosome-like nanoparticles can exert good bioactive functions in different aging or disease models. Thus, we issued the hypothesis that Gouqi-derived nanovesicles (GqDNVs) may also have the ability to improve skeletal muscle health, though the effect and its mechanism need to be explored. Hence, we have extracted GqDNVs from fresh berries of Lycium barbarum L. (goji) and found that the contents of GqDNVs are rich in saccharides and lipids. Based on the pathway annotations and predictions in non-targeted metabolome analysis, GqDNVs are tightly associated with metabolic pathways. In muscle atrophy model mice, intramuscular injection of GqDNVs improves the cross-sectional area of the quadriceps muscle, grip strength and activation of the AMPK/SIRT1/PGC1α pathway. After separately inhibiting AMPK or PGC1α in C2C12 cells with dexamethasone administration, we have found that the activated AMPK plays the chief role in improving cell proliferation induced by GqDNVs. Furthermore, the energy-targeted metabolome analysis in the quadriceps muscle demonstrates that the GqDNVs up-regulate the metabolism of amino sugar and nucleotide sugar, autophagy and oxidative phosphorylation process, which indicates the activation of muscle regeneration. Besides, the results of Spearman rank analysis show close associations between the quality and function of skeletal muscle, metabolites and expression levels of AMPK and SIRT1. In this study, we provide a new founding that GqDNVs can improve the quality and function of skeletal muscle accompanying the activated AMPK/SIRT1/PGC1α signaling pathway. Therefore, GqDNVs have the effect of anti-aging skeletal muscle as a potential adjuvant or complementary method or idea in future therapy and research. (Fig. 1).
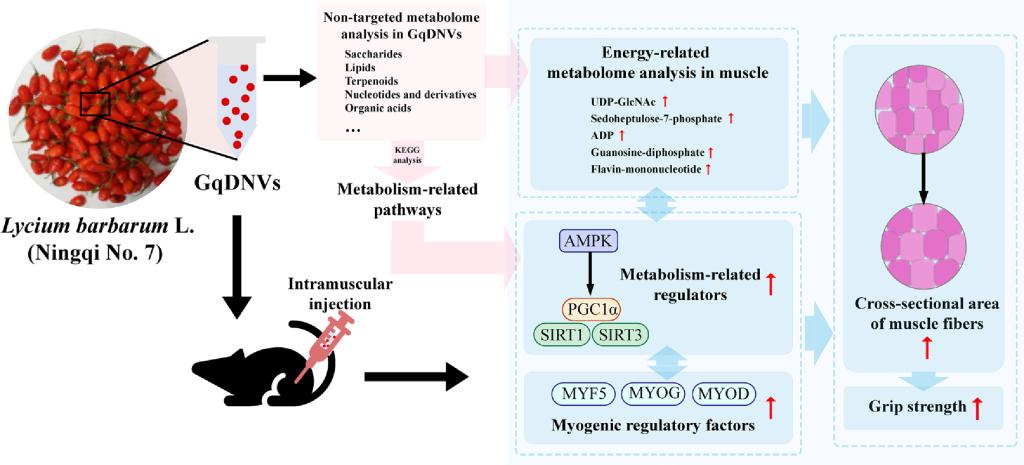
Fig. 1 Graphic abstract of the effect and mechanism of GqDNVs on alleviating skeletal muscle atrophy in mice.
2:Human milk extracellular vesicles enhance muscle growth and physical performance of immature mice associating with Akt/mTOR/ p70s6k signaling pathway (Journal of Nanobiotechnology, 2023).
Extracellular vesicles (EVs) play an important role in human and bovine milk composition. According to excellent published studies, it also exerts various functions in the gut, bone, or immune system. However, the effects of milk-derived EVs on skeletal muscle growth and performance have yet to be fully explored. Firstly, the current study examined the amino acids profile in human milk EVs (HME) and bovine milk EVs (BME) using targeted metabolomics. Secondly, HME and BME were injected in the quadriceps of mice for four weeks (1 time/3 days). Then, related muscle performance, muscle growth markers/pathways, and amino acids profile were detected or measured by grip strength analysis, rotarod performance testing, Jenner-Giemsa/H&E staining, Western blotting, and targeted metabolomics, respectively. Finally, HME and BME were co-cultured with C2C12 cells to detect the above-related indexes and further testify relative phenomena. Our findings mainly demonstrated that HME and BME significantly increase the diameter of C2C12 myotubes. HME treatment demonstrates higher exercise performance and muscle fiber densities than BME treatment. Besides, after KEGG and correlation analyses with biological function after HME and BME treatment, results showed L-Ornithine acts as a “notable marker” after HME treatment to affect mouse skeletal muscle growth or functions. Otherwise, L-Ornithine also significantly positively correlates with the activation of the AKT/mTOR pathway and myogenic regulatory factors (MRFs) and can also be observed in muscle and C2C12 cells after HME treatment. Overall, our study not only provides a novel result for the amino acid composition of HME and BME, but the current study also indicates the advantage of human milk on skeletal muscle growth and performance (Fig. 2).
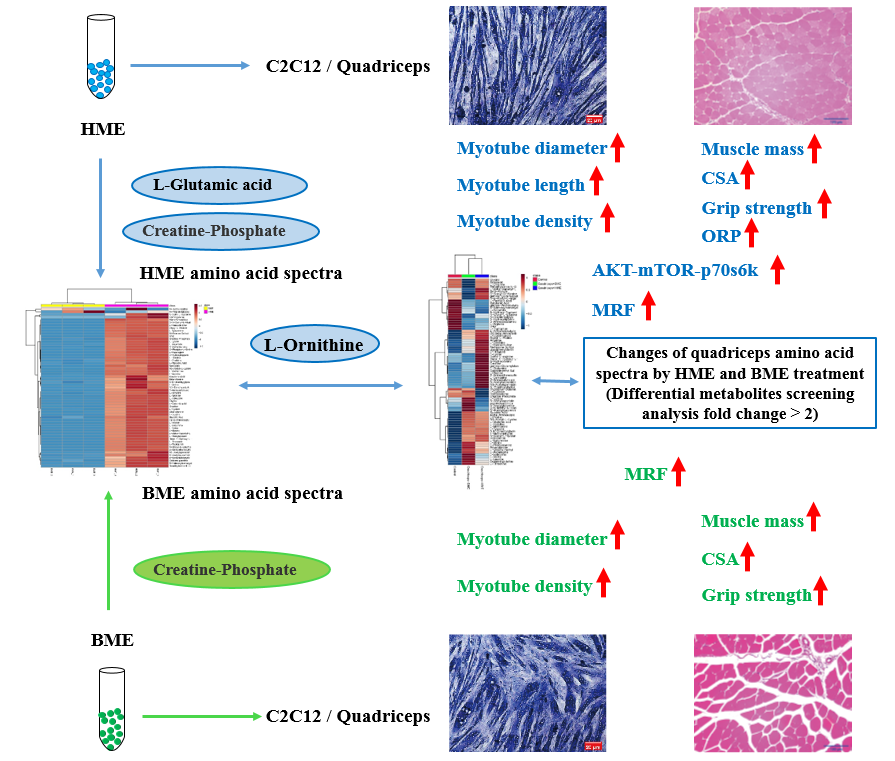
Fig. 2:Graphic abstract for human (HME) /bovine (BME) extracellular vesicles regulates muscle development and aging.
3:Association of muscle wasting with mortality risk among adults: A systematic review and meta-analysis of prospective studies (Journal of Cachexia, Sarcopenia and Muscle, 2023)
The relationship between muscle wasting and mortality risk in the general population remains unclear. Our study was conducted to examine and quantify the associations between muscle wasting and all-cause and cause-specific mortality risks. PubMed, Web of Science and Cochrane Library were searched until 22 March 2023 for main data sources and references of retrieved relevant articles. Prospective studies investigating the associations of muscle wasting with risks of all-cause and cause-specific mortality in the general population were eligible. A random-effect model was used to calculate the pooled relative risk (RR) and 95% confidence intervals (CIs) for the lowest versus normal categories of muscle mass. Subgroup analyses and meta-regression were performed to investigate the potential sources of heterogeneities among studies. Dose-response analyses were conducted to evaluate the relationship between muscle mass and mortality risk. Forty-nine prospective studies were included in the meta-analysis. A total of 61,055 deaths were ascertained among 878,349 participants during the 2.5 to 32-year follow-up. Muscle wasting was associated with higher mortality risks of all causes (RR = 1.36, 95% CI, 1.28 to 1.44, I2 = 94.9%, 49 studies), cardiovascular disease (CVD) (RR = 1.29, 95% CI, 1.05 to 1.58, I2 = 88.1%, 8 studies), cancer (RR = 1.14, 95% CI, 1.02 to 1.27, I2 = 38.7%, 3 studies) and respiratory disease (RR = 1.36, 95% CI, 1.11 to 1.67, I2 = 62.8%, 3 studies). Subgroup analyses revealed that muscle wasting, regardless of muscle strength, was significantly associated with a higher all-cause mortality risk. Meta-regression showed that risks of muscle wasting-related all-cause mortality (P = 0.06) and CVD mortality (P = 0.09) were lower in studies with longer follow-ups. An approximately inverse linear dose-response relationship was observed between mid-arm muscle circumference and all-cause mortality risk (P < 0.01 for nonlinearity). Muscle wasting was associated with higher mortality risks of all causes, CVD, cancer and respiratory disease in the general population. Early detection and treatment for muscle wasting might be crucial for reducing mortality risk and promoting healthy longevity (Fig. 3).
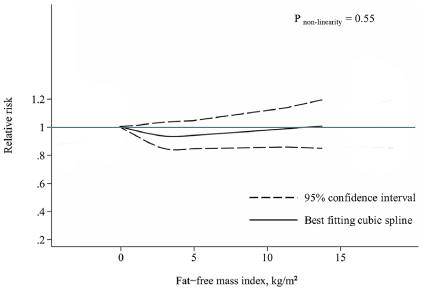
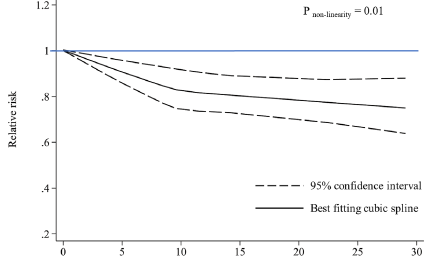
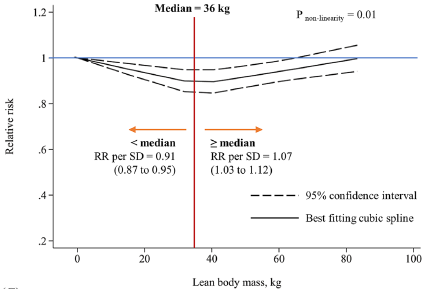
Fig. 3 Dose-response associations between mid-arm muscle circumference, lean body mass, and fat-free mass index with all-cause mortality.
4. Competing endogenous RNA networks were associated with fat accumulation in skeletal muscle of aged male mice (Mechanisms of Ageing and Development, 2024)
Muscle aging contributed to morbidity and mortality in the elderly adults by leading to severe outcomes such as frailty, falls and fractures. Post-transcriptional regulation especially competing endogenous RNA (ceRNA) mechanism may modulate the process of skeletal muscle aging. RNA-seq was performed in quadriceps of 6- month-old (adult) and 22-month-old (aged) male mice to identify differentially expressed ncRNAs and mRNAs and further construct ceRNA networks. Decreased quadriceps-body weight ratio and muscle fiber cross-sectional area as well as histological characteristics of aging were observed in the aged mice. Besides, there were higher expressions of atrogin-1 and MuRF-1 and lower expression of Myog, Myf4 and Myod1 in the quadriceps of aged mice relative to that of adult mice. The expression of 85 lncRNAs, 52 circRNAs, 10 miRNAs and 277 mRNAs were significantly dysregulated in quadriceps between the two groups, among which two ceRNA networks lncRNA 2700081O15Rik/circRNA_0000820-miR-673-3p-Tmem120b were constructed. Levels of triglycerides and expression of PPARγ, C/EBPα, FASN and leptin were elevated and the expression of adiponectin was reduced in quadriceps of aged mice compared with that of adult mice. LncRNA 2700081O15Rik/circRNA_0000820-miR-673-3p-Tmem120b were possibly associated with the adipogenesis and fat accumulation in skeletal muscle of age male mice (Fig. 4 a-c and Fig. 5 a-h).
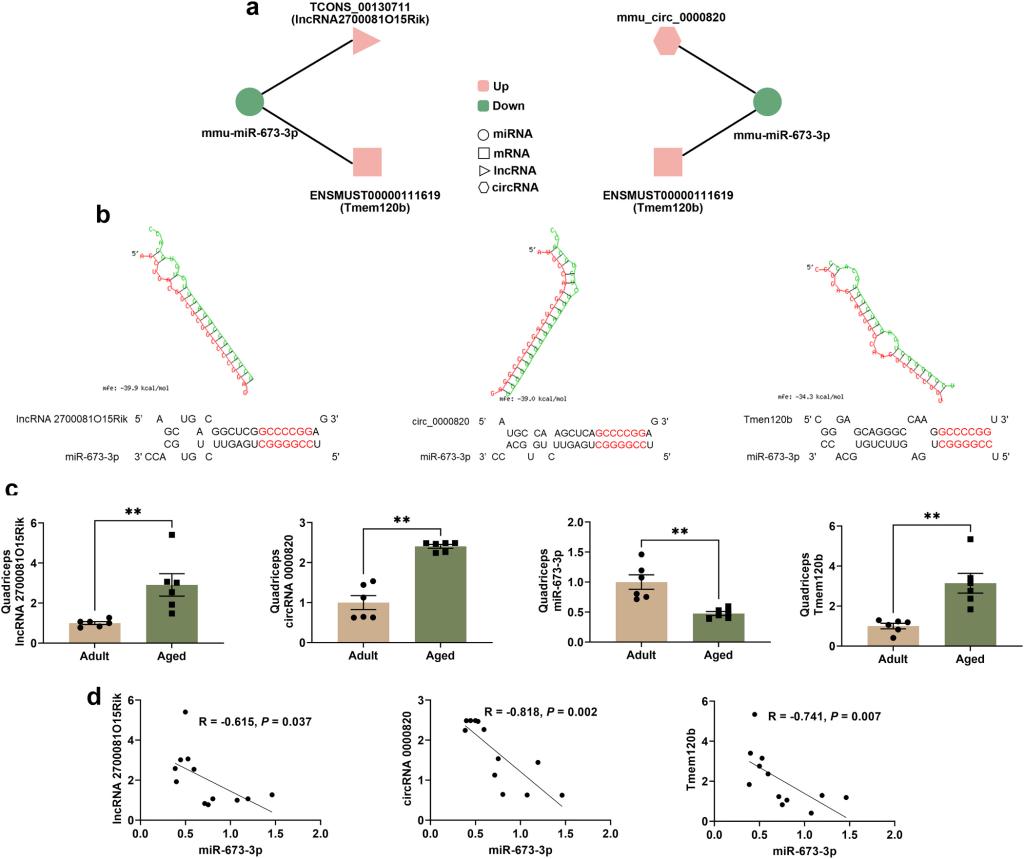
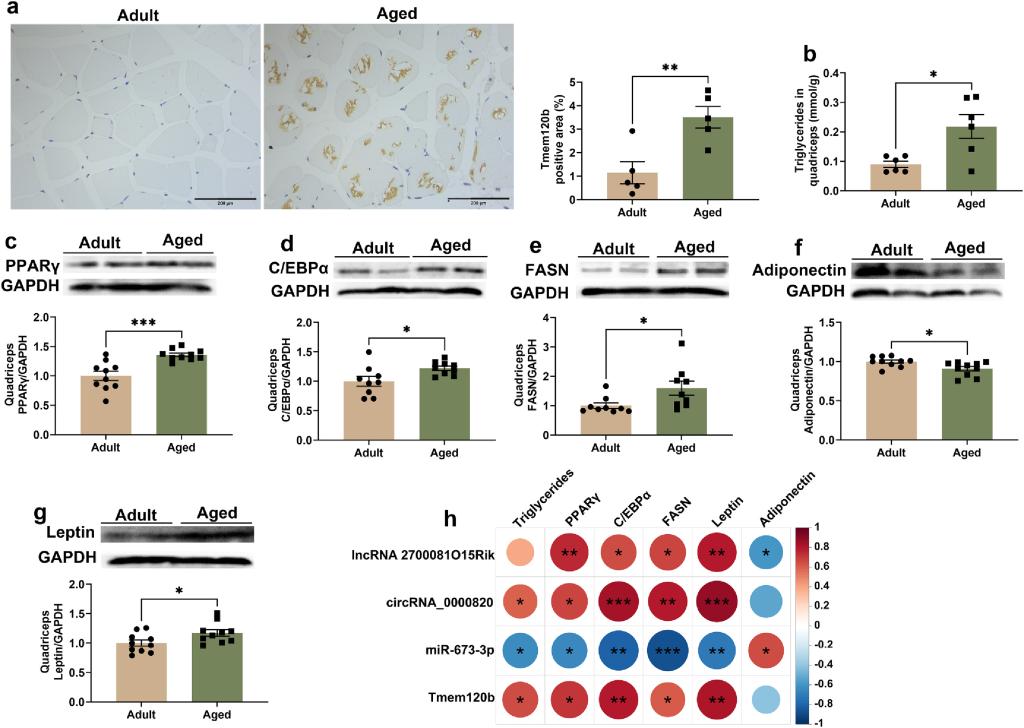
Fig. 4 (Left). Verification of ceRNA networks by RT-qPCR. (a) ceRNA networks lncRNA 2700081O15Rik/circRNA_0000820-miR-673-3p-Tmem120b (P-adjust < 0.05: lncRNA, miRNA and mRNA; P < 0.05: circRNA). lncRNA 2700081O15Rik (TCONS_00130711)/mmu_circ_00008820 and tmem120b (ENSMUST00000111619) shared a common binding site of mmu-miR-673-3p (b) Binding sites predicted by RNAhybrid. mfe: Minimum free energy. The red nucleotides are the complementary sequences to miR-673-3p seed sequences. (c) The expression of lncRNA 2700081O15Rik, mmu_circ_00008820, mmu-miR-673-3p and tmem120b in quadriceps verified by RT-qPCR, n = 6. (d) Spearman correlation analysis between mmu-miR-673-3p and lncRNA 2700081O15Rik/mmu_circ_00008820/tmem120b. ** P < 0.01. Fig. 5 (Right). Fat accumulation in quadriceps of adult and aged mice. (a) Immunohistochemistry of tmem120b in quadriceps (magnification: 400×; scale bar: 200μm, n = 5). (b) Triglycerides levels in quadriceps, n = 6. (c-g) The protein levels of PPARγ, C/EBPα, FASN, adiponectin and leptin in quadriceps, n = 9-10. (h) Spearman correlation analysis between transcripts and fat-related indicators. * P < 0.05; ** P < 0.01; ***P < 0.001.
References:
[1] Xiaolei Zhou, Shiyin Xu, Zixuan Zhang, Mingmeng Tang, Zitong Meng, Zhao Peng, Yuxiao Liao, Xuefeng Yang, Andreas K. Nussler, Liegang Liu, Wei Yang*. Gouqi‑derived nanovesicles (GqDNVs) inhibited dexamethasone-induced muscle atrophy associating with AMPK/SIRT1/PGC1α signaling pathway. Journal of Nanobiotechnology (2024) 22:276.
[2] Zitong Meng, Dong Zhou, Dan Lv, Quan Gan, Yuxiao Liao, Zhao Peng, Xiaolei Zhou, Shiyin Xu, Penglong Chi, Zhipeng Wang, Andreas K. Nüssler, Xuefeng Yang, Liegang Liu, Dongrui Deng, Wei Yang*. Human milk extracellular vesicles enhance muscle growth and physical performance of immature mice associating with Akt/mTOR/p70s6k signaling pathway. Journal of Nanobiotechnology. 2023 Aug 29; 21(1): 304.
[3] Huan-Huan Zhou, Yuxiao Liao, Zhao Peng, Fang Liu, Qi Wang, Wei Yang*. Association of muscle wasting with mortality risk among adults: A systematic review and meta-analysis of prospective studies. Journal of cachexia, sarcopenia and muscle. 2023 2023 Aug;14(4):1596-1612.
[4]. Yuxiao Liao#, Zhao Peng#, Xiaolei Zhou, Huanhuan Zhou, Zitong Meng, Shiyin Xu, Taoping Sun, Andreas K. Nüssler, Wei Yang*. Competing endogenous RNA networks were associated with fat accumulation in skeletal muscle of aged male mice. Mechanisms of Ageing and Development 220 (2024) 111953.

